Abstract

Background Hemophagocytic lymphohistiocytosis (HLH) in adults is a clinical challenge due to its rapid progress and high mortality. The current first-line treatment regimen such as HLH-94 or HLH-2004 are largely extrapolated from pediatric HLH; however, the prognosis of adult HLH is worse than that of children. Ruxolitinib, an oral JAK inhibitor, has shown favorable efficacy and safety in murine models and clinical trials in adult HLH. Moreover, ruxolitinib can reverse the resistance of CD8 positive T cells to dexamethasone in hypercytokinemia of HLH. We were very interested in these results and preliminarily revealed the high response rate and good short-term survival of ruxolitinib combined with dexamethasone (Ru-D) in a pilot study involving 8 adult HLH patients. Based on these previous data, we conducted this prospective, single-center, single-arm, Phase 2 clinical study (ChiCTR2100049996).
Methods This clinical trial aims to evaluate the efficacy and safety of Ru-D regimen as first-line therapy in adults with newly diagnosed HLH. Inclusion criteria were patients who met HLH-2004 diagnostic criteria, older than 18 years old, and had not received HLH first-line therapy. Patients in this study received an 8-week period of treatment. The Ru-D regimen consists of ruxolitinib (15 mg twice daily for 8 weeks) and dexamethasone (initially 10 mg/m2 for 2 weeks followed by 5 mg/m2 for 2 weeks, 2.5 mg/m2 for 2 weeks, 1.25 mg/m2 for one week, and one week of tapering). The primary endpoint was a 2-month survival rate (expected to reach 75%), and the sample size was 28 cases. The secondary endpoints are overall response rate (ORR), progression-free survival (PFS), safety and changes in biomarkers.
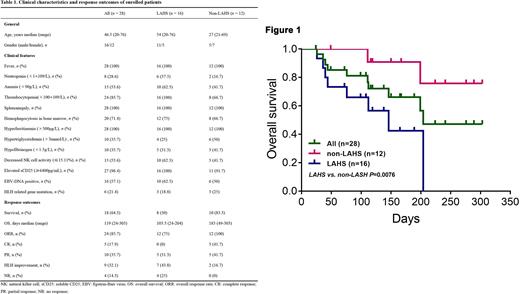
Results From August 15, 2021 to June 21, 2022, there were 28 patients enrolled in this study. Of these 28 patients, 16 cases (57.1%) were eventually diagnosed as lymphoma-associated HLH (LAHS), 6 cases were Epstein-Barr virus-associated HLH (EBV-HLH), 2 cases were macrophage activation syndrome (MAS), and the remaining 4 had unknown etiology. It should be mentioned that 21.4% patients (6/28) carried heterozygous HLH-related gene mutations. The main clinical characteristics of the patients enrolled are summarized in Table 1.Patients were followed up until July 15, 2022 or until death, with none lost to follow-up. The median follow-up time was 119 (24-303) days. The primary endpoint, 2-month OS, was 84.6% in 26 evaluable patients. The overall mortality rate was 35.7% (10/28), 80% (8/10) deaths occurred in LAHS patients until data cutoff. The OS of LAHS patients were worse than those of non-LAHS patients (50.0% vs 83.3%, p=0.0076, Figure 1); however, 93.8% (15/16) LASH patients successfully bridged to chemotherapy after Ru-D therapy. The ORR to Ru-D regime was 85.7% (24/28), with 17.9% (5/28) in complete response (CR), 35.7% (10/28) in partial response (PR), 32.1% (9/28) in HLH improvement, and 14.3% (4/28) in no response (NR). The treatment was well tolerated, with no toxicities leading to dose reduction or discontinuation of treatment.
Conclusion In our study, the Ru-D regimen showed favorable efficacy in newly diagnosed adult patients with HLH. In non-LAHS patients, Ru-D regimen resulted in sustained response in 83.3% patients. For patients with LAHS, it is an efficient and safe bridging therapy while waiting for pathological diagnosis. Therefore, the Ru-D regimen represents a novel and promising therapeutic option for HLH patients that may provide clinical benefits.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Ruxolitinib was use in our clinical trial to treat adult patients with Hemophagocytic Lymphohistiocytosis. The current indications of Ruxolitinib is myelofibrosis.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal